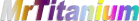 's Introduction to Anodizing Titanium
's Introduction to Anodizing Titanium
This article assumes that you already have an anodizer, and just need some hints on how to use it
Note: This process is different from that used to anodize aluminum.
For that technique (type 2 anodizing for aluminum), try this detailed page on the internet WayBack machine
(because it's not at focuser.com anymore, but it's the one I used way back when MrTitanium went online).
For that technique (type 2 anodizing for aluminum), try this detailed page on the internet WayBack machine
(because it's not at focuser.com anymore, but it's the one I used way back when MrTitanium went online).
The basic process is simple:
- Use rubber gloves to avoid electrocution. Follow all normal high-voltage handling guidelines.
- You need an electrolyte solution in a non-conducting container.

Former Future U.S. President Ronald Reagan Hawking a brand of Borax (not known at that time to be a good titanium anodizing electrolyte)-
TSP (trisodium phosphate), ammonium-phosphate, and Borax are among the chemicals I've used.
TSP is found in the paint department (for washing walls). Ammonium phosphate is a lawn fertilizer.
Borax is found in the laundry aisle (non-chlorine bleach).
It's not really critical what chemical or how strong,
but avoid chlorides, nitrates and sulphates. For either TSP or Borax, about 5g/l is a good concentration (about 1/10 of saturated).
Remember: The water is what does the work; the salt or acid is just an ion source.
NOTE: Environmentally sensitive people worry about TSP and the environment. The electrolyte is not consumed. Several years of anodizing would release as much phosphate into the environment as a single load of laundry or a single treatment of ChemLawn. TSP is only bad if it gets into the aquifers: Just don't pour it down the drain! Save it for reuse, or dilute and spray it on the garden. - I use a Rubbermaid® storage container because they resist chemicals well, last a long time, have lids, and are available everywhere.
-
TSP (trisodium phosphate), ammonium-phosphate, and Borax are among the chemicals I've used.
TSP is found in the paint department (for washing walls). Ammonium phosphate is a lawn fertilizer.
Borax is found in the laundry aisle (non-chlorine bleach).
It's not really critical what chemical or how strong,
but avoid chlorides, nitrates and sulphates. For either TSP or Borax, about 5g/l is a good concentration (about 1/10 of saturated).
Remember: The water is what does the work; the salt or acid is just an ion source.
- For the Cathode (negative electrode): Use a scrap piece of metal immersed in the solution.
I use a loosely coiled titanium wire, but anything non-rusting should work.
Rule of thumb: The immersed surface area of the cathode should be greater than that of the anode (work piece).
In use, hydrogen will bubble off of this piece. For large pieces, make sure you have adequate ventilation to avoid an explosion hazard. - The Anode (+) side is critical. All the parts that are immersed on this side must be titanium, niobium, or tantalum.
Other metals, like stainless or tinned alligator clips simply suck current away from what you are trying to do.
I have made myself an assortment of hooks and clips out of titanium, and ReativeMetals sells some nice electronics clips converted to niobium. - Attach the piece to be colored, the work piece, to the anode (positive electrode). It must be free of oils. I often wipe things down with acetone or alcohol to make sure they are clean. Don't allow any non-titanium parts of the anode circuit to touch the solution. I often lower my pieces into the solution on a titanium hook, or with titanium tongs (both homemade).
- After the piece is immersed, adjust the rectified, regulated, (protected, fused) voltage to get the color you want (between 25-120 vdc).
- Color is voltage dependant. For bigger pieces, it just takes longer. Or more current. Use fuses, an ammeter, and/or a power resistor (or a light bulb in series) to avoid burning out your anodizer.
- Color Guide: The exact voltage it takes to get a particular color depends on many
variable factors such as free-ion content of the electrolyte, surface finish of the metal, etching,
stability of the voltage source, and so on. If you want 2 pieces to exactly match, anodize them at the same time.
 As a rule of thumb, the spectrum is that shown in
the MrTitanium logo. The first tinge of bronze
appears at around 10 volts, the near-white blue is around 50 volts, and the bright green is around 110.
There is no true red, and the dark blues and violets are sensitive to fingerprints.
See my Physics page for more details about how and why the colors work.
As a rule of thumb, the spectrum is that shown in
the MrTitanium logo. The first tinge of bronze
appears at around 10 volts, the near-white blue is around 50 volts, and the bright green is around 110.
There is no true red, and the dark blues and violets are sensitive to fingerprints.
See my Physics page for more details about how and why the colors work. - Never let the anode and cathode pieces touch each
other; short-circuit arc welding is a technique not covered here.
