 (tries to) Explain
(tries to) Explain
Why Titanium is Lightweight
Titanium gets its odd combination of light and strong from its underlying physics. Titanium weighs about 4½ times as much as water, iron and steel weigh about 8 times as much, lead 11½, and gold weighs over 19 times as much as water. "Water?"", you say. The density or "specific gravity" indicates how much mass is packed into a certain volume. Water is a convenient standard against which to measure everything else.
The graph below shows a plot of the Atomic Mass (how much each atom weighs), and Density
plotted against the Atomic Number (how many protons it has).
Notice that, although the density does increase with the weight of the elements, a more important factor is
where is fits in the periodic table; how many free electrons are in the outermost shell.
This periodicity is better illustrated at
WebElements.com.
Play with the different views till you find one that makes sense to you.
In brief: Titanium atoms are relatively light, and their electron shell is moderately wide,
which means that fewer atoms can be packed into a fixed volume.
Notice in the chart how the metal Lead has 5% more mass per atom than
Gold, yet Gold weighs 70% more per cubic centimeter than Lead.
Iron (the mass of steel alloys) has only a slightly heavier atom than Titanium, but its electron shells fit more tightly together. Therefore, Titanium is more than proportionately lighter.
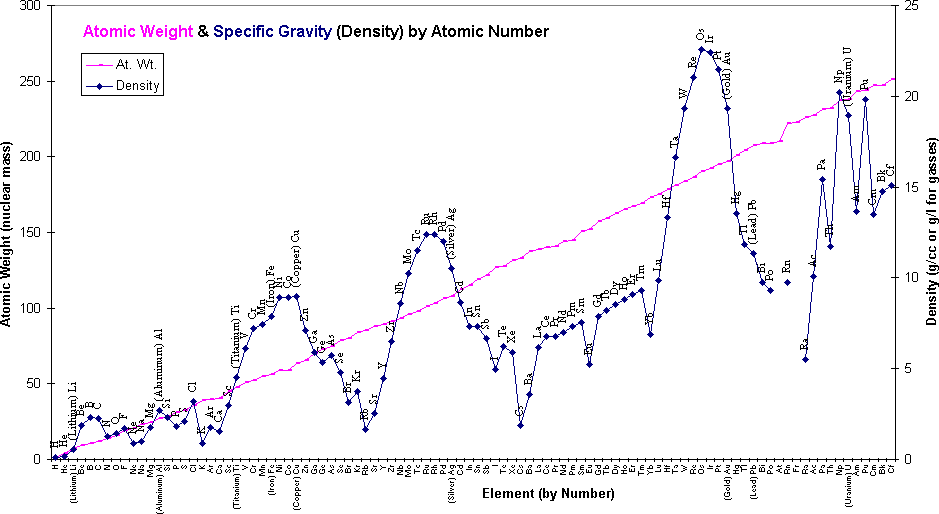
This chart is based on information believed to be correct. I've put the full name of certain metallic elements that I believe are of particular value for comparison and illustration. Gaps in the data represent elements (Promethium, Astatine) for which no isotope stays around long enough to be able to get a density reading in a lab.